The pediatric patient responds to medications differently than the adult patient for a number of reasons.
If one examines the uptake and distribution of medications whether they are administered intravenously, orally, by inhalation, intramuscular injection or rectally, the pediatric patient has the same rapid uptake (alpha phase) followed by the slower, elimination phase (beta phase) as an adult. The timing of these phases may be and often is altered. Generally, these changes relate to: body composition, protein binding, cardiac, renal, and hepatic function.
If one considers the neonate in particular, not only do they look “different” on the outside compared with a more mature infant they also “look different” on the inside. For example if one examines total body water, in a premature infant this accounts for approximately 85% of the patient’s weight. In a term infant this is about 75%, whereas in the 6 month old and older, the total body water only accounts for 60% of body weight (Figures 1 & 2). Likewise, there are marked changes in fat and muscle content as the infant matures.
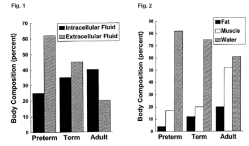
These differences in body composition have important implications regarding drug effect, loading dose, interval of dosing and drug metabolism. For example, if one is giving a medication that is highly water soluble and therefore rapidly redistributed in this large water compartment, then one might have to give a higher initial dose on a mg/kg basis compared with older patients. A good example of this would be succinylcholine and many antibiotics.
In addition, there is a difference in muscle mass. A premature infant has only about 18% of their weight in the form of muscle, a term infant about 30%, a 6 month old about 40% and then most children a year of age or older, approximately 50% of their body weight is in the form of muscle mass. If one is giving a medication that has its primary effect at the myoneural junction, one could postulate that a lower dose would be required or a lower plasma level would be required to have a clinical effect in the infant compared to the older child.
In addition, there are major differences in fat content. A premature infant has only about 4% of their body weight in the form of fat; the term infant has about 14 or 15%; the 6 month old about 25% and older children nearly 30%.
Obviously, this is quite variable among adults. If one has a drug that primarily redistributes into fat tissue, then the volume of tissue into which that drug can be distributed is different according to different ages. For example, thiopental (whose effect is diminished through redistribution rather than metabolism) might have a prolonged duration of action (prolonged sedation) in the premature or term infant, compared to the older patient, just simply on the basis of not having very much fat tissue to redistribute into (Figure 3). In these infants the only place that has fat is the brain!
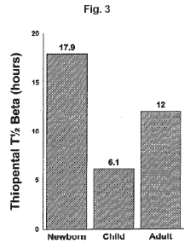
Another difference between infants and older children is maturity of hepatic function. Certainly, newborns are capable of conjugating and glucuronidating most medications but the rate of metabolism is generally delayed compared to the older child or adult. The half-life of thiopental is approximately 18 hours in a term newborn, about 7 hours in a child 4 to 10 years of age and 10 hours in the adult. Thus in addition to having less fat for the thiopental to redistribute into there is a markedly prolonged beta elimination phase due to immaturity of hepatic metabolism. Another factor is alterations in hepatic blood flow and therefore alterations in drug delivery to the liver where it can be metabolized. Any procedure that results in increased intra abdominal pressure such as an omphalocele or gastroschisis repair will decrease hepatic blood flow and also markedly delay drug metabolism. In some neonates with these surgical problems, a single dose of fentanyl has been demonstrated to maintain constant plasma values for as long as 16 hours.
Another issue that markedly alters a drug’s half-life is maturation of renal function. Clearly there is a very rapid maturation of renal function that takes place in the first several months of life (Figure 4).
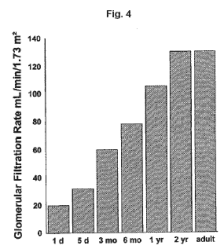
In the preterm infant, the glomerular filtration rate in ml’s/minute/1.73 meters squared surface area is only about 25, in the term infant it is about 35, by two weeks of age it is doubled to approximately 60, by 6 months of age it is 80 ml/minute/1.73 meters squared surface area and at 1 year of age it is roughly equivalent to that of the adult. This is the reason why drugs that are rapidly excreted through the kidneys, such as antibiotics, are given at less frequent intervals in the premature compared to the term newborn and at less frequent intervals in the term newborn compared to the older child.
In addition to these maturational factors in body composition and maturation of renal and hepatic functions, differences in protein binding and competitive drug binding with bilirubin in jaundiced infants may also alter drug metabolism and drug pharmacodynamics. As an infant matures there are marked changes in both total protein and albumen values (Figure 5).
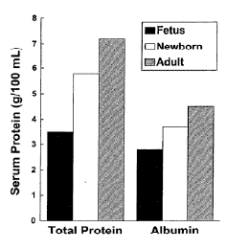
These differences can then alter the amount of drug that is protein bound which in turn affects the amount of free drug available to cross a biologic membrane. Less protein or less albumen results in higher free drug levels.
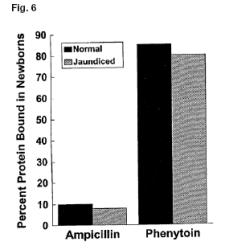
Thus the preterm and term neonates might be particularly vulnerable to drugs effects simply on the basis of altered protein binding. If a drug has low protein binding then this effect is minimal. In the figure below(Figure 6) for ampicillin the change from 90 % unbound drug to 91 or 92% unbound drug in the presence of hyperbilirubinemia is insignificant. However, if a drug is highly protein bound and that drug also competes with bilirubin for the same binding site, then the jaundiced infant may have a marked increase in free drug levels and therefore altered response due to the increase in unbound drug. Thus as illustrated in the figure below the fraction of unbound diphenylhydantoin nearly doubles compared with a fractional change in unbound ampicillin.
Another issue regarding pharmacodynamic differences is the central nervous system and the maturity of the blood brain barrier. For medications with high fat solubility, the blood brain barrier does not make a significant different but it will make a difference for medications that have a low fat solubility since it is fat solubility that determines a drugs ability to cross cell membranes. If one looks at the fat solubility of the synthetic opioid fentanyl compared to morphine, one would find that fentanyl is so fat soluble (nearly 2,000,000 times > morphine!) that there virtually is no blood brain barrier effect. Whereas with morphine, which has a low fat solubility, a proportionally higher amount of morphine would cross into the brain of a newborn compared to an older patient simply because of this difference in fat solubility. This same effect is also very marked for the benzodiazepines. A number of studies have shown that the peak pharmacodynamic effects of benzodiazepines are directly proportional to their fat solubility. Thus the peak EEG effect of diazepam is
nearly three times faster than that of midazolam. A common misconception is the reverse, i.e., because midazolam is shorter acting it must enter the CNS more apidly (see Figure 7).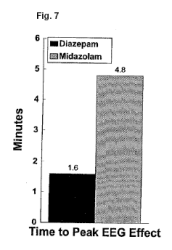
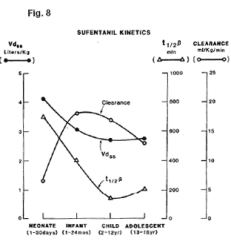
The figure below (Figure 8) illustrates the overall interactions between maturation of excretory mechanisms, alterations in body composition and their effects upon drug clearance and beta elimination half-life for sufentanil.
Transdermal drug delivery
Conclusions
In general, we can say that infants are different from children and children are different from adults. There tends to be a lower ability to excrete drugs in infants because of the differences in glomerular filtration and hepatic function and there are also differences in the way drugs are redistributed within the body, all of which will affect the patient’s ability to eliminate the drug. As the child matures, the ability to excrete and metabolize improves resulting in a shortened half-life of most medications. As we become older, particularly as we become octogenarians, our ability to excrete drugs becomes almost like that of a neonate.